Overview of research
Our research focuses on deciphering the signal transduction pathways in T-cells, and the way by which these pathways control cellular and immune functions in cancer and immunotherapy. Our laboratory was the first to discover the CD4 and CD8-p56lck complexes, the initiators of the phosphorylation and activation cascade in T-cells. A more recent focus has been on the composition of the 'inside-out' pathway that controls T-cell adhesion and the movement of T-cells in lymph nodes, a pathway involving phospho-tyrosine substrates, adhesion- and degranulation-promoting adapter protein (ADAP) and src kinase-associated phosphoprotein (SKAP1). The interaction between SKAP1 and the adaptor RapL accounts for the 'stop signal' needed for T-cells to stop and interact with antigen-presenting cells. We are manipulating the expression of mutant RapL (unable to bind to SKAP1) to stop T-cells for enhanced responses to foreign antigen and neo-antigens in tumors.
Recently, we uncovered a novel pathway involving the adaptor SLP-76 binding to RanGAP1 of the cytoplasmic filaments of the nuclear pore complex (NPC). The interaction controls >50% of T-cell receptor-induced transcription factor (NFAT-c1 and Rel-A) entry into the nucleus of T-cells. A single mutation in a single adaptor that disrupts NPC transport can inhibit T-cell responses by >80%.
A second major area of interest concerns the mechanisms by which co-receptors such as CD28, PD-1 and CTLA-4 control immunity. These receptors modify signals from the antigen-receptor with differences in the threshold of signaling and nature of cytokine production. Our early work identified CD28 coupled pathways involving phosphatidylinositol kinase 3 (PI 3K) and Grb-2. These pathways regulate T-cell proliferation/survival and cytokine production and further motifs that bind to them has been used in chimeric antigen receptor (CAR) therapy in cancer immunotherapy.
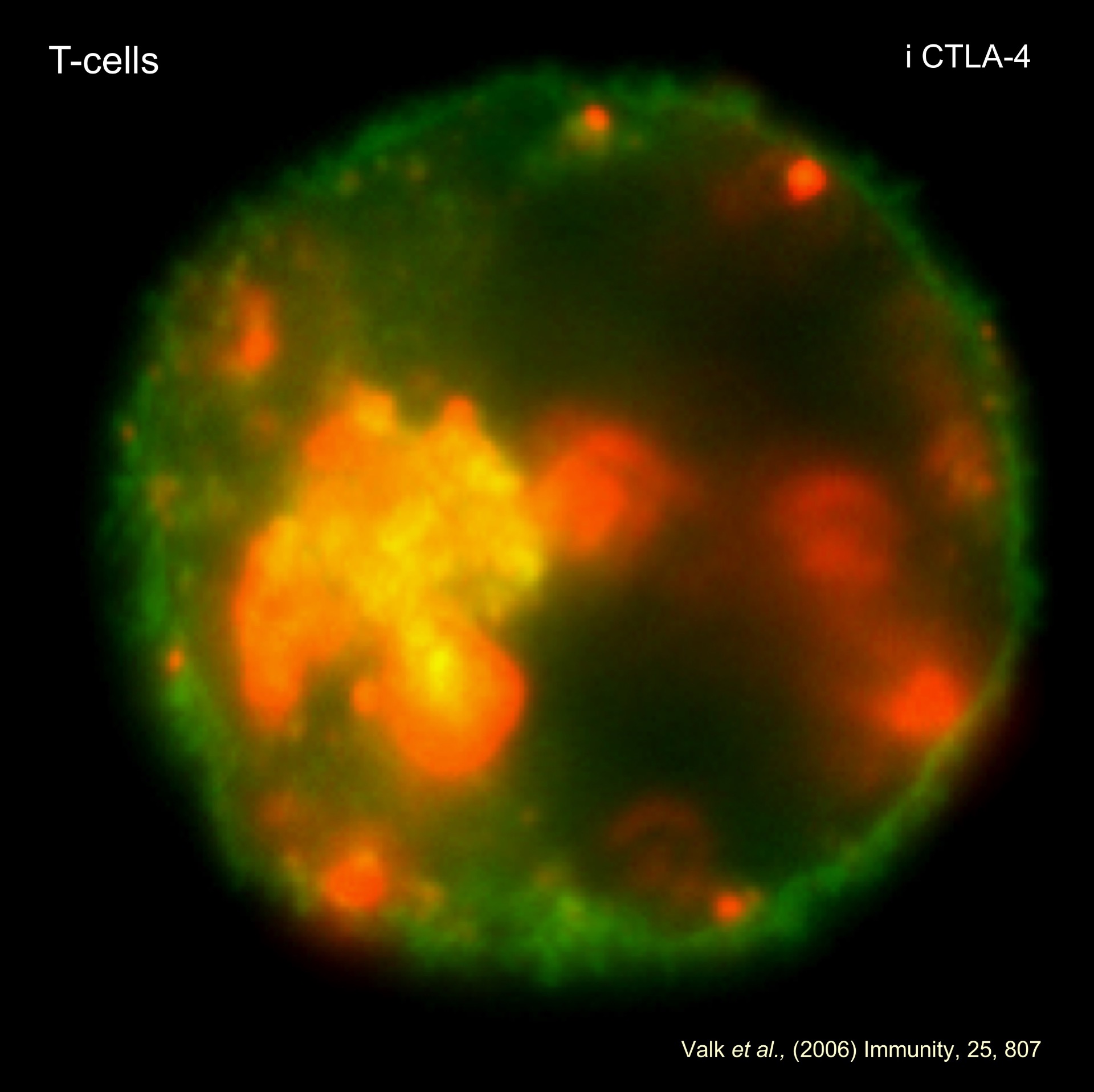
In the case of the negative regulator CTLA-4, many cell intrinsic and extrinsic models exist to explain its function. It is likely that most contribute to its function. We have defined the 'reverse stop signal model' where CTLA-4 activates T-cell motility and thereby limits contact with dendritic cells.
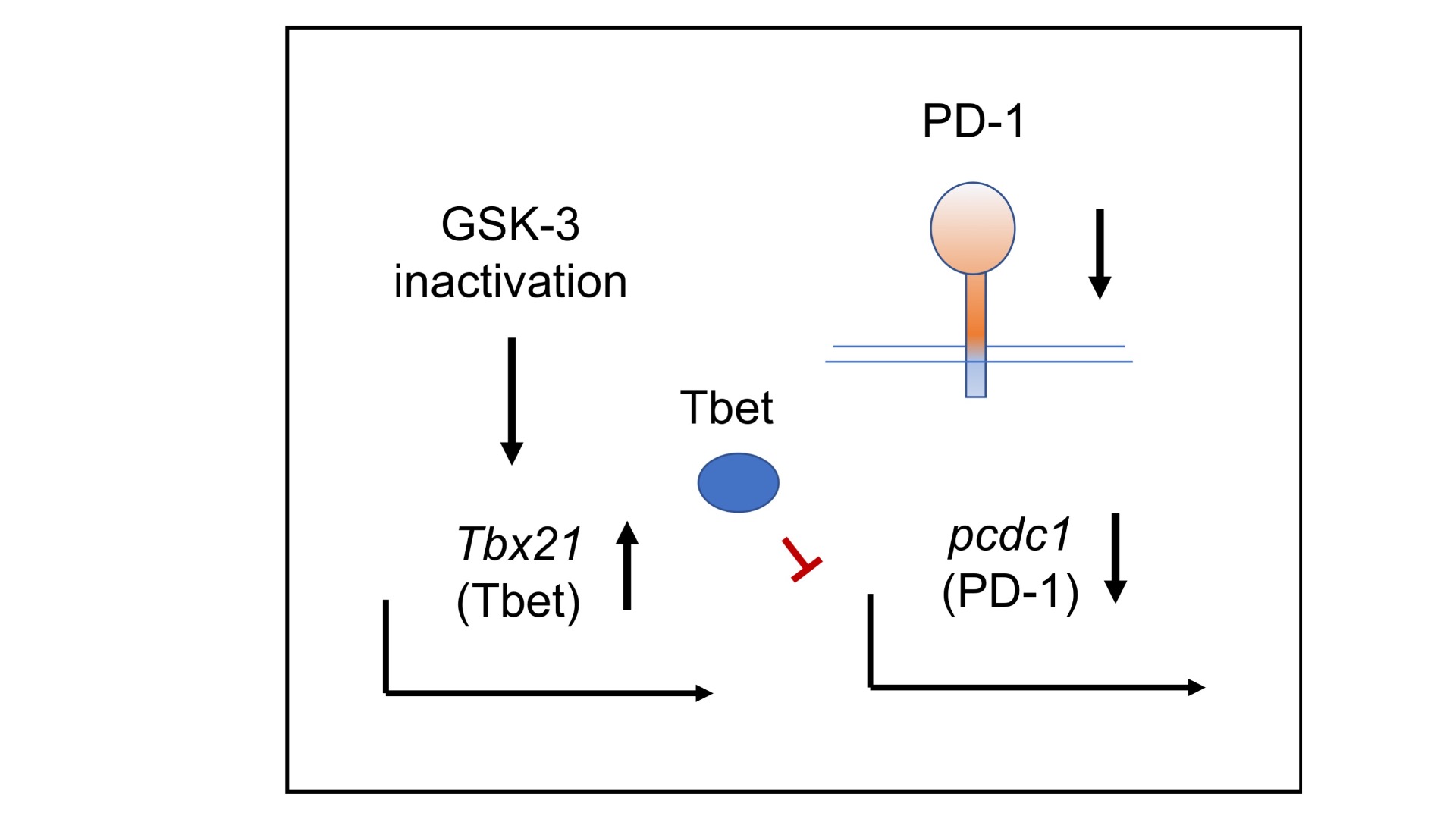
We also recently showed that the serine/threonine kinase glycogen synthase kinase-3 (GSK-3) is a central regulator of PD-1 expression on T-cells and has shown the applicability of small molecule inhibitors (SMIs) of GSK-3 in the down-regulation of PD-1 for clearance of viral infections and cancer. GSK-3 SMIs are as effective as anti-PD-1 in the clearance of B-16 melanoma and EL-4 T-cell lymphomas in mice. Our research is helping to uncover the signaling events that control the adaptive immune response and is assisting in the development of therapeutics for treatment of cancer.